The Ultimate Guide to Kratom’s Key Alkaloids and Their Transformation
Kratom (Mitragyna speciosa) has surged in popularity for its unique effects, largely attributed to two main alkaloids: mitragynine and 7-hydroxymitragynine (7-OH). But what exactly are these compounds, and how does mitragynine transform into the much more potent 7-OH? This comprehensive, SEO-optimized guide will break down the science, pharmacology, and chemistry behind this fascinating process.

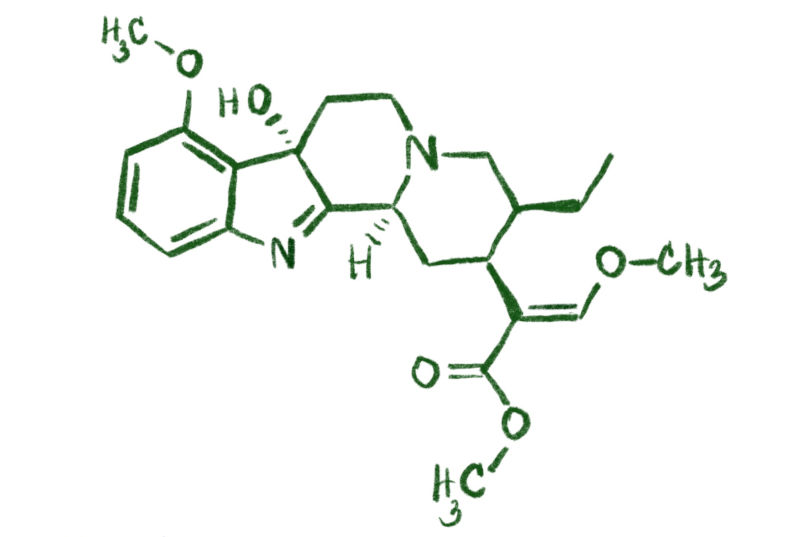
1. What Is Mitragynine?
- Mitragynine is the most abundant alkaloid found in kratom leaves, making up to 66% of the total alkaloid content.
- It is an indole alkaloid with the molecular formula C23H30N2O4 and a molecular weight of 398.50 g/mol.
- First isolated in 1921, mitragynine’s full chemical structure was elucidated in 1964.
- This compound is insoluble in water but dissolves in organic solvents like acetone and alcohols.
- Mitragynine forms white, amorphous crystals with a melting point of 102–106°C.
2. Where Does Mitragynine Come From?
- Mitragynine is naturally produced in the leaves of the kratom tree, which is native to Southeast Asia.
- The leaves are harvested, dried, and either brewed as tea, chewed, or processed into extracts and powders for consumption.
- The concentration of mitragynine can vary depending on the age of the leaves, region, and environmental factors.
3. How Does Mitragynine Work in the Body?
- Mitragynine acts primarily as a partial agonist at the mu-opioid receptor (MOR), which is the same receptor targeted by traditional opioids like morphine.
- It also interacts with delta (DOR) and kappa (KOR) opioid receptors, though its activity at these sites is less pronounced.
- The result is analgesic (pain-relieving) effects, mild euphoria, and, at higher doses, sedation.
- Unlike classical opioids, mitragynine tends to produce milder withdrawal symptoms and less respiratory depression.
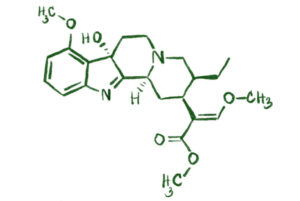
4. What Is 7-Hydroxymitragynine (7-OH)?
- 7-Hydroxymitragynine is a minor alkaloid in kratom, present only in trace amounts in the raw leaves.
- Its molecular formula is C23H30N2O5 (note the extra oxygen atom compared to mitragynine), and its molecular weight is 414.50 g/mol.
- 7-OH is a much more potent agonist at the mu-opioid receptor—in animal studies, it has shown to be several times more potent than morphine as an analgesic.
- This compound is largely responsible for the stronger opioid-like effects sometimes associated with kratom extracts.
5. Why Is the Conversion from Mitragynine to 7-OH Important?
- While mitragynine is the most abundant alkaloid, 7-OH is far more potent at opioid receptors.
- The transformation of mitragynine into 7-OH in the body is believed to be a key reason why kratom can produce strong pain relief and euphoria, even though the raw plant contains very little 7-OH.
- Understanding this conversion helps explain the pharmacological effects and potential risks of kratom use.
6. How Does the Body Convert Mitragynine Into 7-OH?
- The conversion occurs primarily in the liver through the action of specific enzymes.
- The main enzyme responsible is cytochrome P450 3A4 (CYP3A4).
- When mitragynine enters the liver, CYP3A4 oxidizes it, adding a hydroxyl group at the 7-position of the molecule, resulting in the formation of 7-OH.
- This process is known as hepatic metabolism and is similar to how many other drugs are activated or deactivated in the body.
“Mitragynine is converted in vitro in both mouse and human liver preparations to the much more potent mu-opioid receptor agonist 7-hydroxymitragynine and that this conversion is mediated by cytochrome P450 3A isoforms.”
7. What Is the Chemical Process for Converting Mitragynine to 7-OH (Outside the Body)?
- Chemists have developed synthetic methods to convert mitragynine into 7-OH in the lab, often for research purposes.
One common method uses oxidizing agents such as:
- [bis(trifluoroacetoxy)iodo]benzene (PIFA)
- Singlet oxygen
- Potassium peroxymonosulfate (Oxone)
The typical procedure involves:
- Dissolving mitragynine in a solvent (e.g., tetrahydrofuran and water)
- Cooling the mixture and adding the oxidizing agent (e.g., PIFA)
- Allowing the reaction to proceed under controlled conditions (low temperature, inert atmosphere)
- Quenching the reaction and purifying the product using techniques like chromatography
One way to prepare 7-OH, start by dissolving my mitra extract in a mixture of tetrahydrofuran (THF) and water… then add a reagent called PIFA… allow the low-temperature reaction to proceed for 3 to 8 hours.
8. What Are the Key Differences Between Mitragynine and 7-OH?
| Property | Mitragynine | 7-Hydroxymitragynine (7-OH) |
|---|---|---|
| Abundance in Kratom | High (most abundant alkaloid) | Very low (trace amounts) |
| Potency at MOR | Moderate (partial agonist) | Very high (full agonist) |
| Analgesic Effect | Comparable to codeine | Several times more potent than morphine |
| Risk of Dependence | Lower than classical opioids | Higher, similar to potent opioids |
| Formation | Natural in leaves, precursor to 7-OH | Metabolite of mitragynine, synthetic |
9. What Factors Affect the Conversion Rate in the Body?
- Genetic differences in CYP3A4 enzyme activity can affect how quickly and efficiently mitragynine is converted to 7-OH.
- Drug interactions: Some medications inhibit or induce CYP3A4, potentially altering the effects of kratom.
- Liver health: Impaired liver function can slow down or alter the conversion process.
- Dosage and frequency: Higher doses of mitragynine may saturate metabolic pathways, affecting the ratio of mitragynine to 7-OH in the bloodstream.
10. What Are the Pharmacological and Behavioral Effects of Mitragynine and 7-OH?
- Mitragynine:
- Analgesia (pain relief)
- Mild euphoria
- Stimulation at low doses, sedation at high doses
- Milder withdrawal symptoms compared to traditional opioids
7-OH:
- Potent analgesia
- Stronger euphoria
- Higher risk of dependence and withdrawal
Animal studies show tolerance and cross-tolerance with morphine
11. Are There Any Risks Associated With This Conversion?
- The potency of 7-OH means that even small amounts can have strong opioid-like effects.
- Chronic exposure to 7-OH can lead to tolerance, dependence, and withdrawal symptoms similar to those seen with traditional opioids.
- Because the conversion rate can vary between individuals, predicting effects and risks is challenging.
- Combining kratom with other drugs that affect CYP3A4 can increase the risk of adverse effects or overdose.
12. Can This Knowledge Be Used for Safer Kratom Use?
- Understanding the metabolic pathway helps in developing safer kratom products and in anticipating drug interactions.
- It also aids in forensic and toxicological analysis for cases involving kratom.
- Researchers are exploring ways to modulate the conversion to reduce risks and maximize therapeutic benefits.
13. Final Thoughts: The Science Behind Kratom’s Effects
The transformation of mitragynine into 7-hydroxymitragynine is a key factor in the pharmacological profile of kratom. While mitragynine is the most abundant and well-studied alkaloid, its conversion to the much more potent 7-OH—primarily in the liver—explains why kratom can produce strong opioid-like effects, even though the plant itself contains only trace amounts of 7-OH.
As research continues, a deeper understanding of this metabolic pathway will help inform safer use, better regulation, and the development of novel analgesics based on kratom’s unique chemistry.